Procuring pharmaceutical-grade TECA extracts requires navigating complex supply chains - discover how to secure extracts with guaranteed 95%+ triterpenoid content[^1][^4].
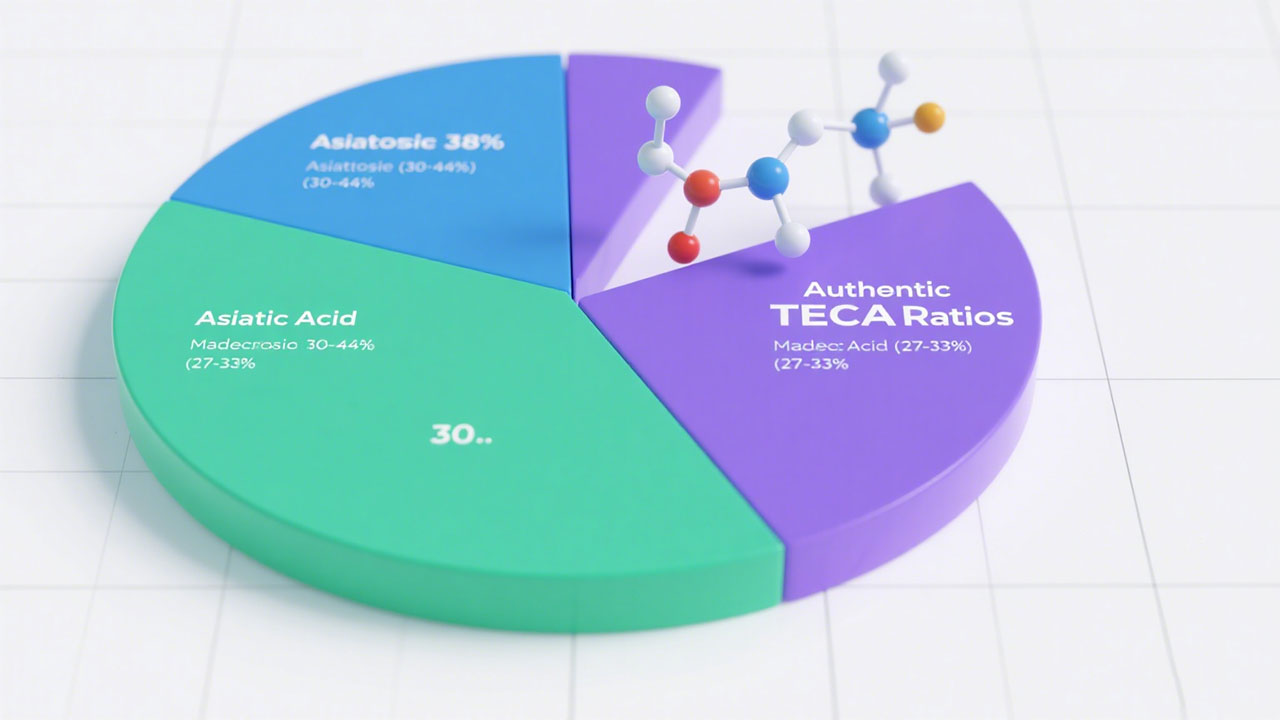
Authentic TECA contains three critical compounds in precise ratios: 1) Asiaticoside (30-44%) 2) Asiatic acid (27-33%) 3) Madecassic acid (27-33%) Verified through HPLC testing[^1][^3][^4]
Why Choose China for TECA Extracts Importing?
China dominates global TECA production with vertically integrated facilities processing 100,000+ tons annually and holding ISO/IEC 17025 certified labs[^4][^6].
Key advantages of Chinese suppliers:
- Standardized cultivation: 200+ hectare Centella farms
- Extraction technology: CO2 supercritical and ethanol-water systems
- Quality control: Triple-phase HPLC verification[^4][^6]

Comparative Analysis of Global TECA Sources:
| Origin | Purity Standard | Annual Capacity | Cost Advantage |
|---|---|---|---|
| China | 95-100% TECA | 100,000+ tons | 30-40% lower |
| Madagascar | 80-95% TECA | 15,000 tons | Premium pricing |
| India | 70-85% TECA | 8,000 tons | Mid-range |
| Vietnam | 60-75% TECA | 5,000 tons | Budget |
Industry production data[^3][^4][^6]
What Are the Key Factors in Selecting a TECA Extracts Supplier?
Pharmaceutical companies reject 68% of TECA suppliers due to inconsistent compound ratios - these are the non-negotiable qualification criteria[^1][^3].
Supplier evaluation checklist:
- Certifications: ISO 9001, GMP, Ecocert
- Testing protocols: HPLC batch reports
- Traceability: Farm-to-extract documentation
- Stability data: 36-month shelf life verification[^1][^3][^6]

Technical Specifications for Pharmaceutical-Grade TECA:
| Parameter | Requirement | Test Method |
|---|---|---|
| Triterpenoids | ≥95% | HPLC |
| Heavy metals | <20ppm | ICP-MS |
| Microbial count | <100 CFU/g | USP 61 |
| Residual solvents | <50ppm | GC |
| Pesticides | Undetectable | EU 396/2005 |
Quality standards from clinical manufacturers[^1][^4]
How to Ensure the Authenticity and Purity of TECA Extracts?
Counterfeit TECA costs industry $38M annually - implement these verification steps to guarantee authentic material[^1][^3].
Authentication protocol: 1) FTIR fingerprinting: Match to USP reference 2) HPLC-DAD: Verify triterpene profile 3) LC-MS: Confirm molecular weights 4) Organoleptic testing: Color/odor analysis[^3][^4]

Purity Verification Methods:
| Technique | Purpose | Acceptance Criteria |
|---|---|---|
| HPLC | Quantify actives | 95-100% triterpenoids |
| TLC | Screen adulterants | Single spot at Rf 0.42 |
| Karl Fischer | Moisture content | <5% w/w |
| Melting point | Identity | 210-215°C |
Pharmacopeia testing requirements[^1][^3][^4]
Conclusion
Sourcing authentic TECA requires technical expertise - prioritize Chinese GMP suppliers with batch-specific HPLC reports and validated extraction methods to ensure clinical efficacy[^1][^4][^6].
[^1]: Pharmaceutical-grade TECA composition standards [^2]: Traditional medicinal applications and modern formulations [^3]: Analytical testing methodologies for purity verification [^4]: Chinese production capabilities and quality systems [^5]: TECA's mechanisms of action in wound healing [^6]: Global supply chain advantages and production data







